Trusted Execution Where It Matters Most.
WHY BIORASI?
For more than 20 years, Biorasi has partnered with sponsors to deliver complex, global trials with confidence and clarity. Our teams work side-by-side with clients to navigate challenges, maintain timelines, and adapt to evolving study needs.
- Embedded, cross-functional teams with clinical and regulatory expertise
- Global reach with flexible operations across 46 countries and 2,000+ sites
- Transparent performance tracking through Beeline dashboards: real-time data, zero guesswork
- A CRO model that scales to your study — and never loses sight of your goals

PROOF OF PERFORMANCE
- 200+ global studies executed
- 5 FDA approvals in seven years, supported across multiple therapeutic categories
- 80% of repeat sponsors return with follow-on work
- Studies conducted in 46 countries, reaching 65% of the global population
THERAPEUTIC AREAS OF EXPERTISE
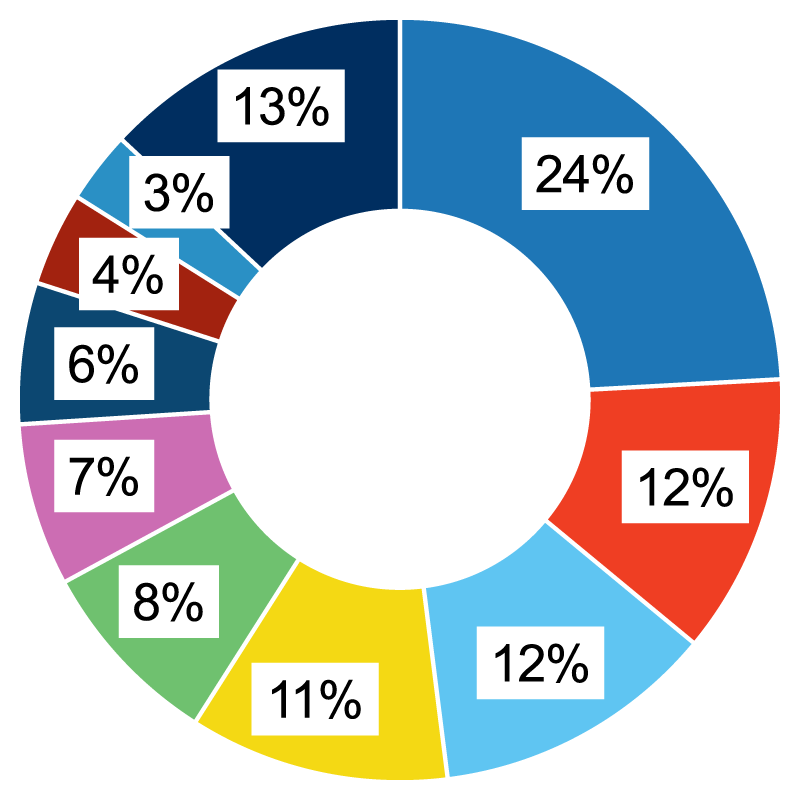
Neurology (24%)
Cardiovascular Disease (13%)
Dermatology (12%)
Gastroenterology (12%)
Oncology (11%)
Ophthalmology (8%)
Infectious Disease (7%)
Pulmonary/Respiratory (6%)
BIORASI GETS RESULTS.
- Global Oncology Rescue: Transitioned within 90 days from incumbent CRO and met all enrollment milestones
- Large-Scale Microbiome Trial: Completed during pandemic with <5% dropout across 140+ patients
- Parkinson’s Program: Delivered integrated regulatory and operational support through to FDA approval
- Cell Therapy Study: Enabled site activation, vendor coordination, and real-time reporting metrics
- Parkinson’s Disease
- Autism Spectrum Disorder
- C3G and IC-MPGN
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
