Biorasi knows that patient safety comes first across all stages of clinical research, from innovation and discovery to clinical trials and clinical practice. Our Safety and Pharmacovigilance team provides end-to-end safety activities designed to meet your study goals for patient care and regulatory compliance.
Comprehensive safety strategies
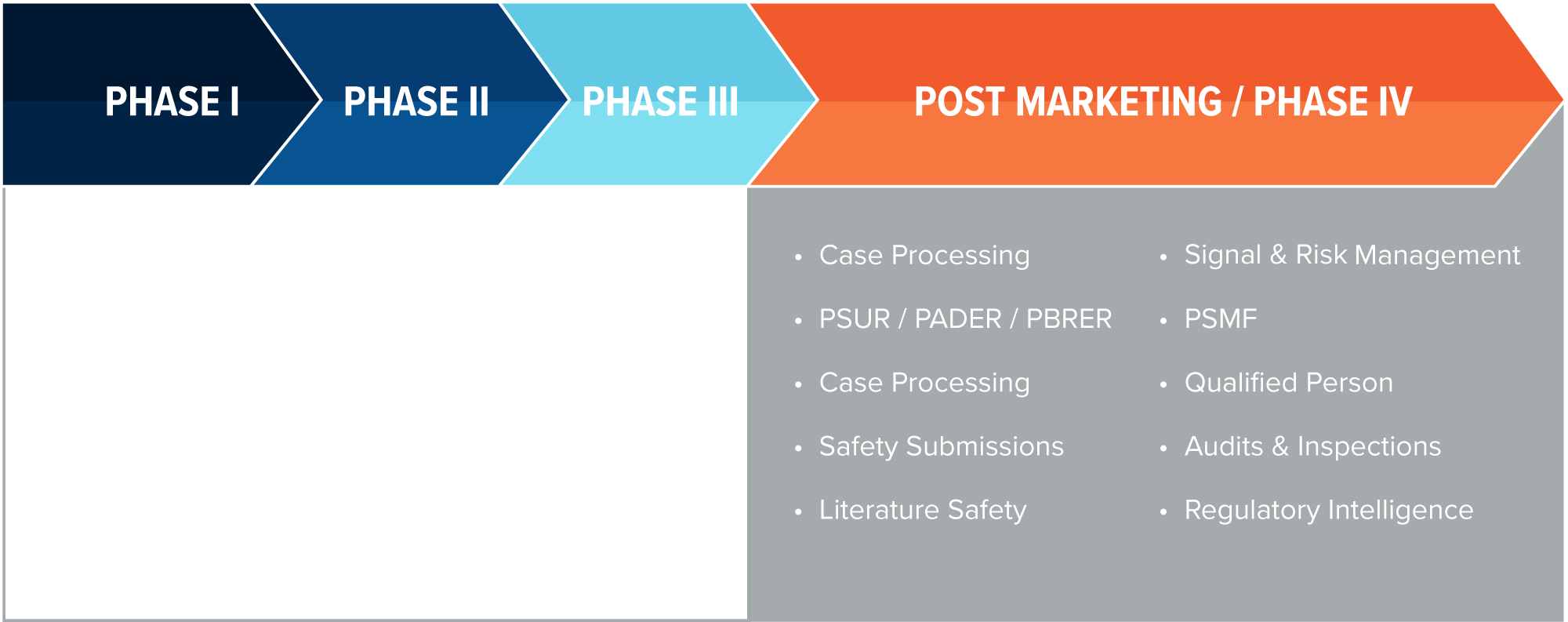
Pre-Marketing/Phase I to III: What are your safety data goals? Biorasi leverages over 20 years of experience in Safety and Pharmacovigilance to give your clinical trials an edge right from the beginning.

Medical Coding
Case Processing
Data Monitoring Committee Management

Safety Management and Annual Reporting
Timeline Compliance
Real-world data capture
Post-Marketing/Phase IV+: Our Safety and Pharmacovigilance expertise doesn’t end outside of the clinical trial setting. Biorasi offers a broad range of post-marketing and surveillance solutions for Phase IV trials and beyond.

Medical Information
- 24/7 Call Handling
- Medical Inquiry Triage

Case-Processing
- Adverse Event Triage
- Data Entry
- Medical Coding
- Medical and Quality Review

Literature Safety Management
- Safety Literature Monitoring
- Social Media Monitoring

Aggregate Safety Reporting
- PBRER
- PSUR
- PADER

Safety Management
- PSMF Management
- SDEA Management

Risk Minimization
- Signal Management
- RMP Management
- REMS Management

PV Quality
- System Audits
- PSMF Audits
- Distributor Audits
- Gap Assessment
- Due Diligence
- QPPV Support

Regulatory Safety
- Pharmacovigilance Regulatory Intelligence
Audit-ready for the world
Standalone & Mock Audits: Biorasi works with you every step of the way throughout your clinical trial. We offer standalone audit services to ensure your team is ready for regulatory compliance and approval. Plus, we can provide mock audit activities to bring your data and reporting in line for upcoming regulatory reviews.
The Qualified GVP Auditors at Biorasi can support pharmacovigilance inspection readiness through:
- Full System Audit
- Distributor / Business Partner Audit
- QPPV Audit
- Affiliate Audit
- PSMF Audit
- Gap Assessment