Resource • Case Study
Case Study: Achieving 15-Day Go-Live for Clinical Trial with EDC/IRT Strategy

Background
With the COVID-19 pandemic’s continued threat to the global population, an estimated 2,000+ pharmaceutical and medical device companies are swiftly working toward developing vaccines and interventions for the coronavirus.
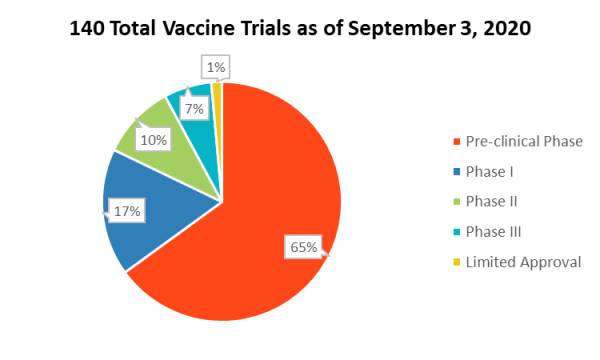
Source: New York Times
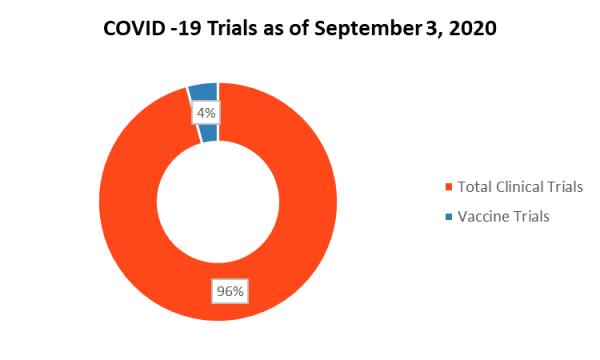
Source: Clinical Trials Arena
Biorasi was engaged by one of its sponsors to manage a clinical trial for treatment of lung injuries derived from the COVID-19 infection.
Drug development and testing for COVID-19 interventions are impacted by a very small window for completion. This is because:
- Patients are in dire need of quick treatment for both disease symptoms and injuries
- Pharmaceutical companies are in direct competition with each other to produce interventions quickly and realize ROI
- Resources and patient availability, including site availability, are becoming more scarce in the speed-to-market race
Biorasi’s client had an aggressive timeline and required a fast and agile CRO. Their goal was to ensure that subjects would be enrolled and treated during the peak of the pandemic in the United States as fast as possible.
One of the primary critical path deliverables in any clinical trial is the development of the EDC (electronic data capture) system. The key challenge was providing an execution strategy for database development that maximized speed, agility, and quality in order to achieve go live. This included:
- EDC eCRFs (electronic case report form), fields, visits, and system rules validation per FDA Guidance for Industry protocols
- 21 CFR 11 compliance with computerized systems used during the clinical trial
- Inclusion of advanced field dynamics and field system queries
- Partner-documented UAT (user acceptance testing) participation
- Deployment and validation of advanced EDC modules, such as randomization and medical coding
This is because:
In order to meet the challenges of this clinical trial, Biorasi utilized an agile development methodology – a software development strategy model that recognizes potential unpredictability and the need for fast development cycles.
“The goal in our repurposing of the agile development methodology was to focus on meeting our sponsor’s clinical trial needs through numerous cycles of refined and increased functionality,” said Raul E. Lopez, Data Management Manager at Biorasi. “We achieved this by optimizing incremental and iterative work sequences called ‘sprints’ and frequent group review sessions, or ‘scrums.’ Coupled with our robust collaboration and communication with the sponsor, this led to a faster and more productive set up for the trial.”
The strategy was powered by two integral solutions
- Leveraging Biorasi’s Global Team: Biorasi was able to deploy concurrent data management teams for this project in the United States and India. This allowed for a 24-hour work cycle, ensuring that the clinical trial set up was always moving forward toward the go-live date and that iterations and feedback could be integrated quickly.
- Utilizing IBM Clinical Development (ICD) CDMS Platform: IBM Clinical Development is a single, fully-integrated, SaaS CDMS designed to help reduce the cycle time to start, amend, and manage clinical studies — enabling life sciences organizations to deliver therapies and innovations faster to their patients.
“At IBM, we’re incredibly proud to partner with Biorasi by providing IBM Clinical Development at no cost to help get COVID-19 treatments to patients who need them as fast as possible,” said Tim Watson, Sr. Business Development Executive at IBM Watson Health. “Biorasi’s incredible achievements in accelerating this critically important trial exemplifies our ultimate goal for our CDMS solution: to reduce the cycle time of clinical studies, enhance the quality of data, and empower life sciences organizations to deliver safe therapies and innovations faster.”
Results
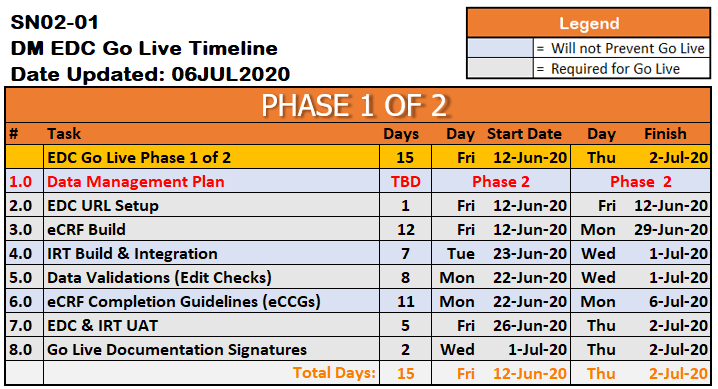
With the intense collaboration between the two Biorasi data teams and the sponsor, as well as the capabilities of the IBM Watson Health ICD platform, Biorasi was able to maximize speed and meet the sponsor’s tight timeline for the clinical trial – also achieving the fastest EDC go live time in the history of the company at 15 days:
15 Days – EDC Ready for Go Live
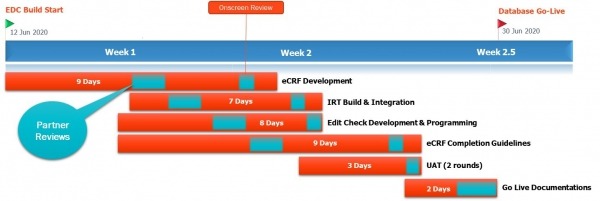
15 Day Timeline
“Biorasi continues to expand on our strategies for leveraging global teams across several concurrent EDC build milestones,” said Roberto Silberwasser, Senior Director of Data Sciences and Biometrics at Biorasi. “These solutions yield faster build times with a focus on the highest level of quality. We look forward to applying these successful integrations into further phases of this clinical trial as well as in future opportunities to boost speed and make patient treatment available faster.”
Biorasi would like to thank the following team members for their dedication during this successful go-live launch:
- Raul E. Lopez, Manager, Data Management (Biorasi U.S. Team)
- Amol Samudre, Manager, Data Management (Biorasi India Team)
- Archana Ghule, Senior, Data Management (Biorasi India Team)
- Remya Sudhir More, Associate, Data Management (Biorasi India Team)